Research
Our lab has a deep and sustained interest in how fungal cell regulates their shape. Our research interests are shaped by the critical roles that fungal morphology and cell wall play in disease progression and antifungal drug targeting. In the last decade, there has been a notable increase in the incidence of invasive mycosis, partly due to a rise in the immunocompromised patient population. Additionally, the range of geographically restricted endemic fungal diseases is expanding, which increases the size of the human populations at risk. These challenges are not limited to human health. White-nose syndrome, an infection in bats by the fungus Pseudogymnoascus destructans, is decimating the bat population in North America. Very little of the cell biology of P. destructans is known. To tackle this challenge, our research program aims to provide a mechanistic understanding of how fungal pathogens respond to anti-cell wall drugs and how fungi regulate their morphology. The cell wall is an attractive therapeutic target since mammalian cells lack this structure, and it provides the fungal cells with mechanical force to withstand the changing environment inside the host. This research program will provide critical insight into fungal cell biology, leading to the development of effective strategies to treat invasive mycoses. For these reasons, our research program aims to provide insights into the following questions:
|
1) Does the septin cytoskeleton contribute to fungal host-pathogen interactions?
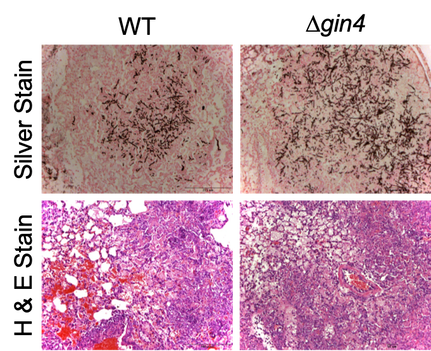
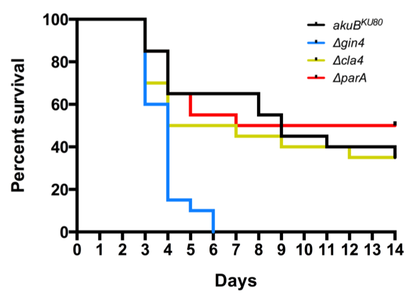
A. fumigatus infections have multiple manifestations, depending on the host’s immune status. Invasive aspergillosis is the most severe type of Aspergillus-related infection, targeting severely immunocompromised patients. The Infectious Diseases Society of America issued a statement that emphasized A. fumigatus as one of six pathogens for which new treatments are critically needed due to the increased incidence of invasive aspergillosis, its excessive mortality rate (35-95%), and the lack of effective treatments. Previous work done by Dr. Vargas-Muñiz during his Ph.D. training defined septins as important cytoskeletal complexes that regulate cellular processes and morphogenesis events in A. fumigatus. His studies elucidated non-overlapping roles for individual septin genes in conidiation, septation, and cell wall stress resistance. Ultrastructural studies implicated the core septin complex in the formation and/or maintenance of the conidial cell wall. Interestingly, the septin deletion strains exhibited hypervirulence in an invertebrate model of invasive aspergillosis and increased TNF-alpha production in bone marrow-derived macrophages. These results point to the importance of A. fumigatus septins as modulators of host-microbe interactions. In a second project conducted by Dr. Vargas-Muñiz, he examined how cells control where septins assemble in space and time. He evaluated how septins might be regulated by mutating a series of septin-associated kinases and phosphatases. Loss of one of these kinases, Gin4, increased the virulence of A. fumigatus in both the invertebrate and murine models due to uncontrolled fungal growth. These results highlight the importance of septin regulators in A. fumigatus development. |
|
2) How the septin cytoskeleton contributes to anti-cell wall drug response?
The anti-cell wall drug caspofungin is guideline-recommended as second-line therapy for invasive aspergillosis after the azoles treatment, which targets the cell membrane components. However, the increasing emergence of azole resistance amongst A. fumigatus isolates is causing an increase in the use of caspofungin to treat invasive aspergillosis. Therefore, it is critical to understand the fungal response to this antifungal agent. Caspofungin targets beta-1,3-D-glucan synthase, which synthesizes a major component of the A. fumigatus cell wall. Caspofungin is fungistatic against A. fumigatus. However, the mechanism behind the A. fumigatus response to this commonly used antifungal is not fully understood. Deletion of septin genes increased the susceptibility to caspofungin in Candida albicans and A. fumigatus, suggesting a broader role of septin-mediated caspofungin response in pathogenic fungi. Nonetheless, the mechanism behind the septin-mediated response to caspofungin remains unclear. |
|
3) What molecular mechanisms do marine fungi use to alter their morphology and adapt to stress?
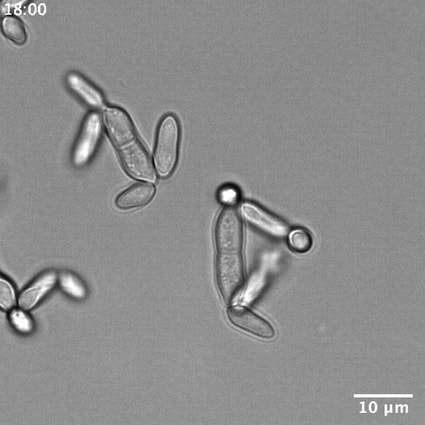
The black yeast Hortaea werneckii is the etiological agent of tinea nigra, a superficial skin infection commonly localized in the palms of the hands or the soles of the feet. Tinea nigra is common in the tropics, including the Caribbean, mainly affecting immunocompromised patients. Additionally, H. werneckii strains have been isolated in challenging environments such as ocean water and marine salterns. H. werneckii displays a striking combination of division patterns, indicating that rules for the cell cycle established in conventional model systems may be broken by fungi growing in extreme environmental conditions. Cell size is highly variable, suggesting cell cycle regulation may have different mechanistic roots than the well-studied model yeasts. Specifically, these cells alternate between fission and budding from cell cycle to cell cycle, which is highly distinct from the conventional model yeast systems. The cell division patterns and morphology of H. werneckii can be regulated by the concentration of NaCl. Morphology is also highly variable between isolates of H. werneckii from diverse environments, making it a prime candidate for comparative genomics to identify the molecular mechanisms that control different cell morphologies. 4) How do Pseudogymnoascus destructans invade host tissue?
P. destructans is the etiological agent of white-nose syndrome, a cutaneous disease responsible for the rapid decline in the North American bat population. We have minimal knowledge of P. destructans cell biology and what mechanism it uses to invade host tissue. Invasion of host tissue is a crucial step in infection establishment and a prime candidate for mitigating P. destructans infection in hibernating bats. We are currently developing genetic and cell biology tools to get a mechanistic understanding of how P. destructans invade host tissue. We are also developing new genetic screening approaches to identify possible drug targets to inhibit this essential pathogenic process. |